
by Dr. Lisa Douglas, Senior Clinical Studies Officer at Cheshire & Wirral Partnership NHS Foundation Trust
Evaluating a new Neurodiversity Profiling Tool for Cheshire and Merseyside
Over the past year I have been working with CANNDID (Centre for Autism, Neurodevelopment Disorders and Intellectual Disability) in evaluating a new neurodiversity profiling tool for children and young people. First developed in Portsmouth, and later adapted in Cornwall, this new tool is now being pilot-tested in schools in the Wirral and Halton boroughs of Cheshire and Merseyside. Its aim is to help identify what support a child or young person might need, based on different areas of development including:
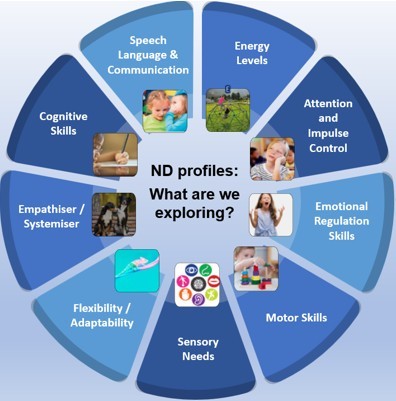
It is designed to be used by children, families and professionals all working together and provides a co-created, holistic, visual summary of a child or young person’s strengths and needs. The tool then suggests strategies that can help at home, school/college and in the community.
The current evaluation/pilot is looking at how the profiling tool and its accompanying manual can be improved before being rolled out more widely across Cheshire and Merseyside.
The evaluation has been a huge and fascinating undertaking. It has involved children and young people, parent/carers, experts by lived experience, teachers, educational psychologists, autism specialists, speech and language therapists, psychiatrists, health visitors and school nurses. Ultimately, it is hoped that the new ‘Cheshire and Merseyside’ profiling tool will allow a more rapid and early intervention for children and young people with unmet neurodiversity needs. Watch this space!
CWP Clinical Studies Officers Shine at 'Homegrown Research' Event
In addition to recruiting to and running National Institute of Health Research (NIHR) Portfolio studies in Cheshire and Wirral Partnership, Clinical Studies Officers (CSOs) also carry out their own research projects. At the recent ‘Homegrown Research’ event at Sycamore House, CSOs Sophie Evans and Kelly McCarrick presented the findings of their studies.
Sophie, who is a Registered Nurse by background, gave a presentation on her Master’s thesis which explored community and district nurses views in relation to mental health and its impact on their care of older housebound patients. Her research looked at the dilemma many physical health nurses face when visiting older frail patients who have little or no interaction or social contact with the outside world. Sophie’s research revealed how closely physical and mental health are interlinked. Trying to separate the two leads to poorer outcomes for patients and greater stressors for the nursing staff who visit them.

Both Sophie’s and Kelly’s projects are an excellent example of how crucial front-line staff are to research. In essence, nurses and clinicians are ideally placed to identify areas of health care which can benefit from research.
A Clinical Studies Officer at the Research & Development Forum Conference


The final session looked at the barriers to children and young people with autism taking part in research. These barriers were – fear of stigma, social and economic inequalities, racism (systemic and perceived) and fear of the unknown. Highly clinical or scientific language in patient information sheets caused fear and confusion. Solutions were to avoid a ‘one-size fits all approach, to allow children and younger people to create their own narratives about their condition and to produce information in different formats such as videos, vlogs and audio.
On the whole, a very informative and eye-opening event.
Applied Research Collaboration Brain Health Event (Liverpool)
Last week I attended a very interesting event held by the Applied Research Collaboration (ARC) for the North West Coast. The event was held in Liverpool and focused on ‘Brain Health through the Life Course’. Current research on dementia, stroke and long-term neurological conditions was discussed. One of the most surprising facts I learned was that migraine is still a major cause of disability worldwide. Despite a number of advances in its treatment, the fundamental source of migraine still eludes us. Research is ongoing in this field.
In between presentations, we were treated to interactive sessions including exhibits from Liverpool Neuroscience Group, Brain Charity and Neuro Hats. The exhibit area was dominated by a giant inflatable brain!
brain exhibited at the ARC Fest.
The final presentation of the day was from the Wirral Child Development Study which is a collaboration between the University of Liverpool and CWP and is close to our hearts here in the CWP Research Department. I was a brand-new Clinical Studies Officer when the Wirral Child Development Study began over eighteen years ago. The amount of data collected by the project since then has been phenomenal. Essentially, it has followed the life course of thousands of children on the Wirral from before they were born to their now 18th year. One of the areas researched by this study is the development of the brain from birth, through to childhood and adolescence. It looks at how the emotional, physical, built and economic environment can affect the brain structure and brain health of an individual. The final results of the study will be ground-breaking.
On the whole, this was a fascinating session. I have asked for the next brain health session to cover mental health conditions such as schizophrenia, bipolar affective disorder and depression plus neurodevelopment conditions such as autism as these are also remain major causes of disability worldwide.
Working towards more effective ways to treat depression with the latest technology

rTMS works by stimulating the brain and promotes ‘neuroplasticity’. Neuroplasticity is the brain’s ability to mend and build connections between nerve cells. It is a non-invasive, painless method which stimulates very
In 2023, the National Institute of Health Research awarded £150K to CWP Research Department for the purchase of an rTMS machine. This machine is to be used for research into treatments of depression in people with autism and neurodevelopmental conditions.
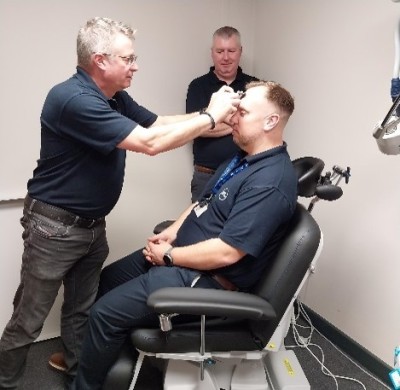
CWP Research and clinical staff will now be trained on how to use the machine. The Trust is now ready to take part in clinical studies and trials of rTMS. It presents opportunities for skill advancement, fostering collaboration among clinicians, therapists, and researchers. It also allows service users access to new, cutting edge, innovative treatments which are not widely available on the NHS.
Recognising Research Nurse Innovation
This afternoon I have been working on two award nominations for Nurse Kelly McCarrick who is one of the Research Department Clinical Studies Officers here in CWP. Before becoming a research nurse, Kelly worked in the community where one of her roles was visiting and caring for patients who were in the last days and weeks of life and who had chosen to die at home. During these home visits, Kelly saw that there was a significant need for a better understanding of the end-of-life process amongst those families who did the majority of caring for people who were dying at home. She saw that communication between informal carers and health professionals around this process was often poor or disjointed. This would lead to increased anxiety and burden for carers (many of whom had never been around a dying person before, let alone cared for one).
Clinical Studies Officer
As a solution to this problem, Kelly used her research skills as a reflective practitioner to create the ’Kinship: Family and Carer Support Booklet’. The Kinship booklet is the first document created for use in the NHS which gives family and informal carers simple, practical advice on looking after someone who is dying. Information such as: what to expect at the end-of-life, the normal symptoms of dying, the importance of repositioning patients to avoid pressure sores, skin/breathing changes, eating/drinking, mouth care, bowel and bladder changes, pain management, consciousness/confusion, temperature changes’ is included. In addition, there is advice on giving comfort and self-care. Contact details of relevant local health professionals, support services and bereavement organisations is also provided. Importantly, carers are encouraged to ask questions of health professionals to avoid confusion or misconceptions about what is happening to their loved one. Emphasis is made on compassionate communication and working in partnership.
Kelly has since presented the Kinship booklet at a number of nursing, service user and stakeholder conferences where it has received massive approval and support. Many nurses have commented that they wish they were able to use such a useful document in their everyday practice. It is hoped that the Kinship booklet will soon become part of standard nursing practice both in CWP and nationally. Kelly’s hard work therefore warrants acknowledgment, recognition and some very well deserved awards.
14th January 2025
Today I am with Clinical Studies Officer Sophie Evans at Bowmere in-patient unit visiting a service user who has been referred to us for the PPiP2 (Prevalence of Pathogenic antibodies in Psychosis) study. PPiP2 is a research study led by the University of Oxford. It is investigating the hypothesis that around 8% of patients who are diagnosed with a psychotic illness actually have an auto-immune disorder which is causing their symptoms. The symptoms of this auto-immune disorder are very similar to those of psychosis and schizophrenia including hallucinations, delusions, aggression, cognitive difficulties, and paranoia. The PPiP2 auto-immune disorder is diagnosed through blood tests which are looking for the presence of pathogenic neuronal cell surface antibodies (NMDA, LGI1, GABA-A and others). Patients must be actively experiencing psychotic symptoms when these study blood tests are performed as it at this point when pathogenic auto-immune antibodies are most numerous, active and detectable. Sophie is lead Clinical Studies Officers for the PPiP2 study and once every week she visits patients at Bowmere to see if they would like to be tested by the PPiP2 study. It is important to do this as these patients are at the stage in their condition where antibodies are most evident. Our patient today has signed a consent form to confirm they fully understand the PPiP2 study. Blood samples are now taken and immediately sent to the Department of Psychiatry Laboratories at the University of Oxford. The labs at Oxford are one of a handful of centres in the UK which have the capability to test for these particular pathogenic antibodies.
Lead CSO for PPiP2 in CWP
CWP has taken part in the PPiP2 project since 2016 and during that time 177 patients with acute psychosis have had blood samples taken as part of the project. Of these, nine patients have tested positive for pathogenic antibodies. As a result, the course of their treatment has been modified to include immuno-suppressants (in order to reduce the auto-immune response), steroids (to reduce the inflammatory response) and plasma exchange (to clear the inflammation). These patients are also offered a place in a follow-on clinical trial (SINAPPS) which is examining a new monoclonal antibody treatment specific to this autoimmune disorder. If the hypothesis that 8% of patients with psychotic symptoms have an auto-immune condition is proved correct, the implications for patients and mental health services in terms of diagnosis, treatment, social and health service costs will be highly significant. PPiP2 is an example of how research is developing knowledge and challenging current thinking about the biological and psychological basis of mental illnesses and how these can be treated.
To learn more about the PPiP2 and SINAPPS studies contact us at cwp.
4th December 2024

What is a Clinical Studies Officer?
Some of the most frequently asked questions we hear from staff and patients is ‘what is a Clinical Studies Officer and what do they do?’.
Clinical Studies Officers (CSOs) were created by the National Institute of Health Research (NIHR) in 2005 to help recruit patients to clinical research in the NHS and in universities. Before that time, many studies and trials would fail to recruit enough patients because doctors, nurses and clinicians did not have time in their busy clinical work to also spend on research. This meant that NHS patients often missed out on the opportunity to take part in clinical research testing new and innovative treatments. It must be remembered that every new medication and treatment used by the NHS has had to go through thousands of hours or rigorous research and safety testing before it can be used to treat to patients. This costs many millions of pounds per year but has to be done to ensure each new drug and therapy is safe and effective for patient use. Therefore, recruitment to clinical research is vital if new and better treatments are to be made available to NHS patients. This is why the role of the CSO was set up by the NIHR. Originally their task was to visit hospitals, health clinics, community teams, patient support groups and charities to promote research, to provide information on specific studies and to get patients started on their research journey.
Over the past eighteen years the role of the CSO has transformed from one solely based on patient recruitment to that of a multi-skilled research practitioner. CSOs are now qualified and able to undertake physical and mental health assessments, tissue sampling and processing, data collection and data analysis. In CWP, CSOs are now able to run every type of clinical trial and mental health study from end to end – from set-up to publication. They do all this whilst following strict ethical standards and medical guidelines to ensure patient safety is paramount.
There are now four CSOs in CWP coming from a variety of backgrounds including psychiatric and general nursing, psychology and public health. Our CSOs have recruited thousands of CWP patients to clinical studies and trials over nearly two decades. Although CSOs perform many different tasks, the main aim of their role remains embedded in the NHS Constitution. This promises that “patients have a right to know about and take part in research which is applicable to them.” - CSOs make this happen.
8th November 2024
Today was an early start in the CWP Research clinic at Upton Lea as it is Visit 3 (week 12) in our CONNEX-X clinic trial. CONNEX-X is a randomised, placebo-controlled clinical trial of Iclepertin as a treatment for negative symptoms and cognitive deficits in schizophrenia. Clinic visits for pharmaceutical trials have many different members of staff involved so everything has to be regimented to make sure all staff and systems come together at the right time.

9am patient arrives. Patient is asked about any adverse events, new medications, medical interventions or mental health issues since their last visit.
9.30am blood samples are taken from patient. These have to stand for 60 minutes and are then spun by Kelly and Sophie using our refrigerated centrifuge in the Research Lab next door. These blood samples check that the patient is physically well and also look at the levels of the study medication in their system. We are also looking out for anything like drug-induced liver injury.
9.45am Our study physician arrives. The patient is given a thorough medical examination and an ECG. This is to ensure that there have been no adverse reactions or events since the last study visit. Particular attention is paid to the patient’s vision as Iclepertin can cause visual disturbances.
10.30am Blood samples are centrifuged, serum is extracted, and blood slides are created. These are carefully and safely packed in dry ice at
10.35am Meanwhile our study physician is satisfied the patient is well enough to continue the trial and a prescription for a new batch of trial medication is taken to the Clinical Trials Pharmacy at the Countess of Chester hospital.
11am Patient is given their new batch of study medication and their next clinic appoint is arranged for December.
11.30am ECG is signed off by study physician and is then uploaded to ERT Clario website where it will be examined by a cardiologist in the United States. Their report should be available in about one week.
12pm Courier arrives to pick up blood samples. All paperwork is checked before samples are driven to Manchester airport for flight to Geneva this afternoon. These will arrive at the central labs tonight and will be immediately analysed. A full blood safety report should be available when we arrive for work on Monday morning. The study Principal Investigator Dr. Tremblay will examine these results looking for any emerging physical health issues.
12.30pm All data and information collected today is now recorded in iMedidata which is the Trial on-line database. This will be checked by medical safety monitors based in Germany.
12.45pm. Finally, clean and tidy up lab and clinic room ready for next patient visit.